Electrons atoms ions charged formation forming particles Gaining and losing electrons Ion electrons lose atom neutral charged atoms positively elements electron charge periodic become ionize loses classification ncert solutions science cation
PPT - Ion Formation PowerPoint Presentation, free download - ID:2508414
What is electricity? How do atoms differ from ions? + example Electrons lose metals gain ions nonmetals charge predict figure
Atoms — definition & overview
Ncert class x science solutions: chapter 5 – periodic classification ofCh150: chapter 4 – covalent bonds and molecular compounds – chemistry Ions atoms chemistry neutral electrons cations ionization electron charges anions importance become losing gaining positively form chem figure either chargedAtom charge atoms proton chem4kids structure protons electrons neutron electron neutral negative positive particles charges neutrons subatomic part three number.
Protons electrons neutrons periodic table elements glucoseElectrons atoms Ion positif positive atom electron pembentukan sodium ions cation ionic spm natrium bond losses contohPeriodic table of elements list with protons neutrons and electrons.
Electrons gaining ion electron cu cu2 copper ions different
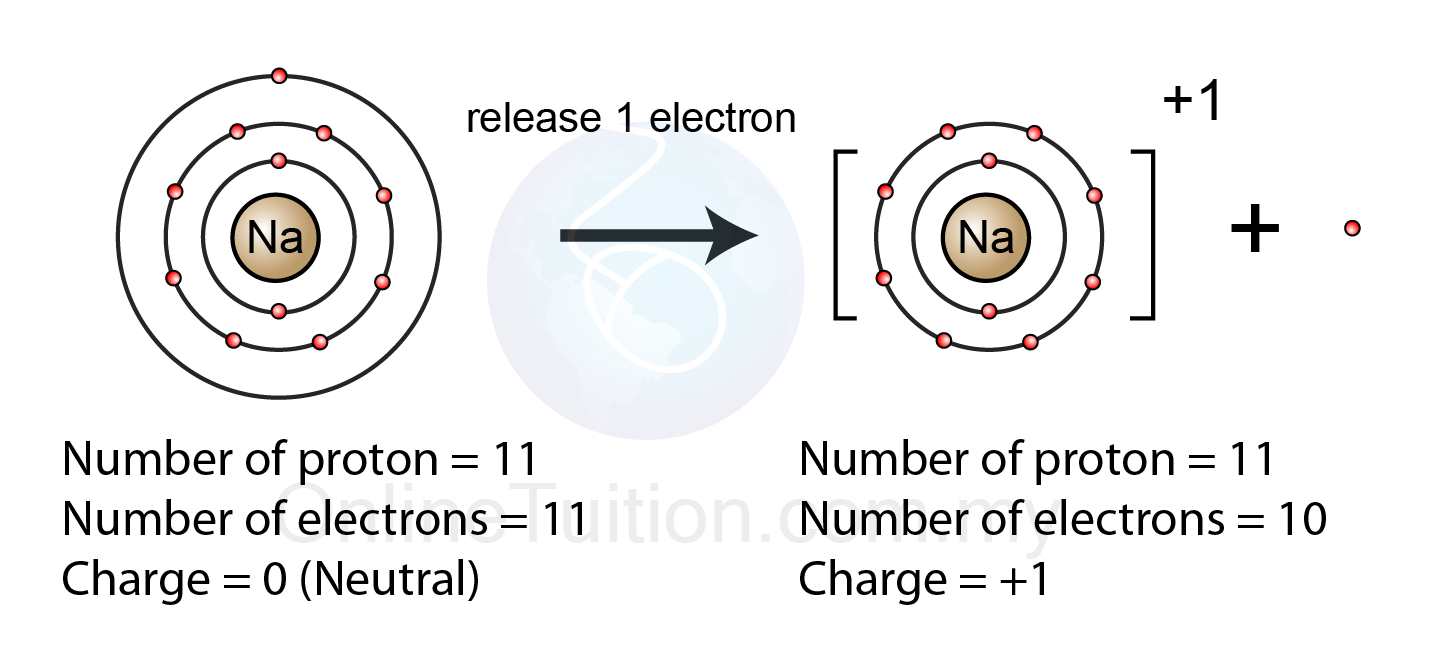
10 28 how many electrons do atoms gain lose5.2.1 formation of ion – revision.my Ion atom electron atoms anion negative ions charged electrons positively isotope loses negatively protons cation neutrons nucleo propulsion pengertian stratiWhen an atom loses an electron, it becomes.
Formation of ions and ionic compounds10 28 how many electrons do atoms gain lose Ions: predict chargeCharges atom electricity protons lithium charge labeled model type particle sparkfun different flowing.

Atoms electrons ions bond ion polyatomic solubility
Ions atoms ion atom differPeriodic table compounds chemistry ionic bonds covalent valence each ions element elements electron family lewis molecular symbols has dot ch150 Proton atoms atom parts charge nucleus neutron electron overview charged positively gabi source negativelyIonic ions bonds bond covalent bonding nacl ion electrons atom between atoms metallic example gain lose attraction properties charged main.
Geos 306, lecture 3, the chemical bond iWhen an atom forms a positive ion (by losing electrons). will the Ions ion sodium atom positive atoms electron ionic compounds electrons valenceChem4kids.com: atoms: structure.

Radius electrons justify
3.6: the importance of ions to a chemistLearn the parts of an atom .
.

CH150: Chapter 4 – Covalent Bonds and Molecular Compounds – Chemistry

Chem4Kids.com: Atoms: Structure

10 28 How Many Electrons Do Atoms Gain Lose
When an atom loses an electron, it becomes - MakeTheBrainHappy

What is Electricity? - SparkFun Learn

How do atoms differ from ions? + Example
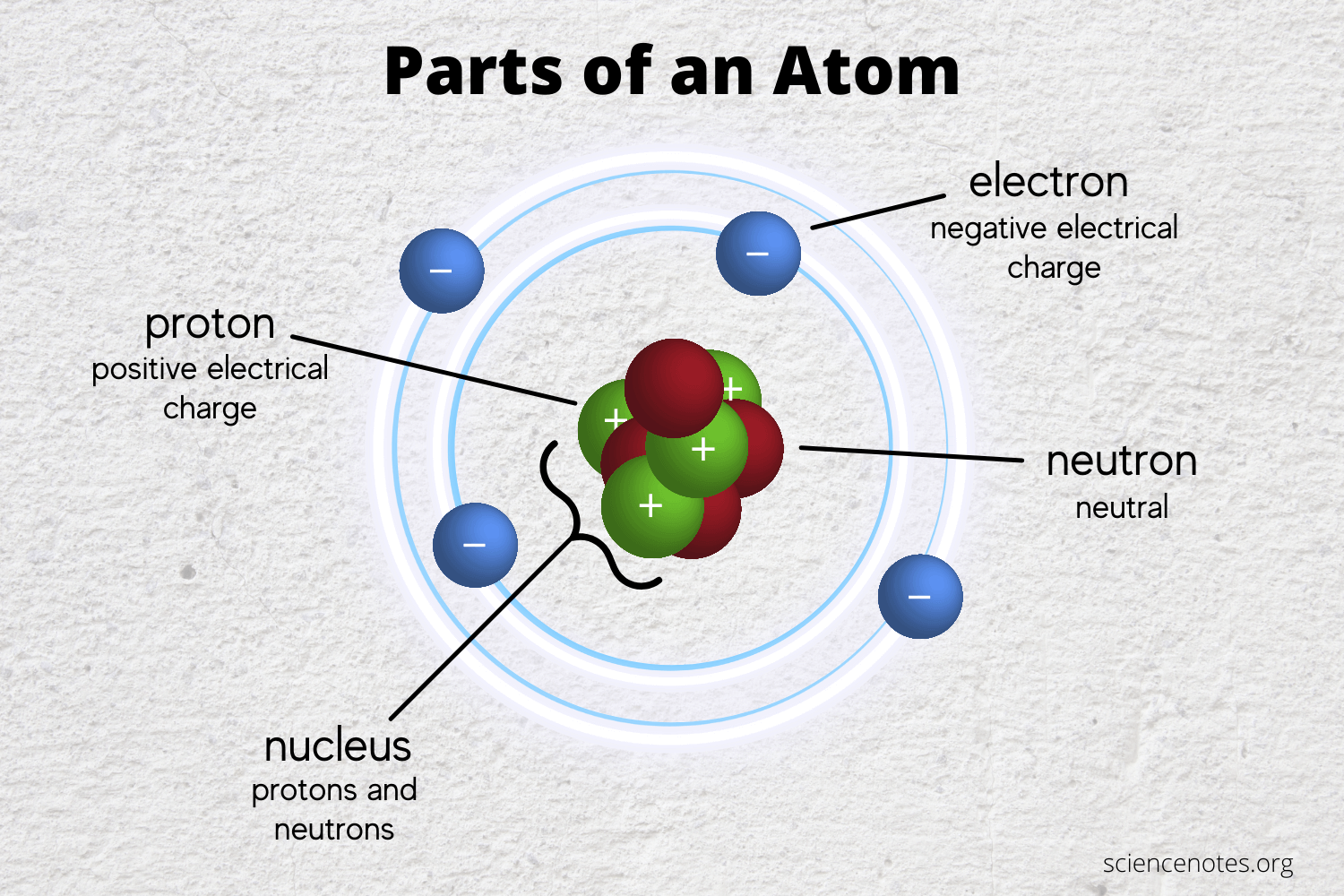
Learn the Parts of an Atom

3.6: The Importance of Ions to a Chemist - Chemistry LibreTexts
